
Washington State Health Care Authority
Manufacturer Data
Submission Guide
Drug Price Transparency – RCW 43.71C
Version 2.0
Effective Date: 3/1/2022

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
1
Contents
About ................................................................................................................................................................................. 3
Contacts ............................................................................................................................................................................. 3
Compliance Questions or General Program Questions..................................................................................................... 3
Technical Support .............................................................................................................................................................. 3
Definitions ......................................................................................................................................................................... 3
Submission Schedule ......................................................................................................................................................... 5
How to Register ................................................................................................................................................................. 5
How to Submit ................................................................................................................................................................... 5
Submission Specifications ................................................................................................................................................. 6
Data Validation .......................................................................................................................................................... 6
Resubmissions ........................................................................................................................................................... 6
Table Specifications ........................................................................................................................................................... 7
New Covered Drugs and Qualifying Price Increases.................................................................................................. 7
New Drug Application.............................................................................................................................................. 18
Appendix A – ST Web Client User Guideline ................................................................................................................... 24
Prerequisites ............................................................................................................................................................ 24
Sign in with your password ..................................................................................................................................... 25
Main page in ST Web Client .................................................................................................................................... 27
Welcome menu ....................................................................................................................................................... 27
Set preferences........................................................................................................................................................ 28
Select a Transfer mode ............................................................................................................................................ 28
Change your password ............................................................................................................................................ 29
Upload files .............................................................................................................................................................. 30
Actions Drop Down Menu ....................................................................................................................................... 30
Download files ......................................................................................................................................................... 30
To create folders...................................................................................................................................................... 31
Uploads monitor Page ............................................................................................................................................. 32
Monitor uploads ...................................................................................................................................................... 32
Information Displayed ............................................................................................................................................. 32
Filter uploads displayed ........................................................................................................................................... 32
Resume uploads ...................................................................................................................................................... 33
Remove display entries ........................................................................................................................................... 33
Appendix B – SFT Client Options (Partial List) ................................................................................................................. 34

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
2
WaTech supported clients- ............................................................................................................................................. 34
Default browser client ............................................................................................................................................. 34
Upload a file by selecting “Browse” tab .................................................................................................................. 34
Download a file ........................................................................................................................................................ 34
Enhanced Browser Client ........................................................................................................................................ 35
Upload a file by selecting “Upload” tab .................................................................................................................. 35
Download a file by ................................................................................................................................................... 36
Optional Clients ....................................................................................................................................................... 36
WinSCP – With Basic setup information and requirements ................................................................................... 36
WinSCP – With Basic setup information and requirements – cont’d ..................................................................... 37
FileZilla- Basic information ...................................................................................................................................... 38
Other client information ......................................................................................................................................... 39

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
3
About
In 2019, the Washington State Legislature passed a law (Chapter 43.71C Revised Code of Washington) which created
the Drug Price Transparency (DPT) program at Health Care Authority (HCA). The law requires issuers of health
insurance, pharmacy benefit managers (PBMs), manufacturers, and pharmacy service administrative organizations
(PSAOs), to submit data on drug costs and pricing to HCA. HCA will use the data to create annual reports that
demonstrate the overall impact that drug costs, rebates, and other discounts have on health care premiums.
You may visit HCA website for more information about the Drug Price Transparency program.
https://www.hca.wa.gov/about-hca/clinical-collaboration-and-initiatives/prescription-drug-cost-transparency
HCA developed this submission guide with input from stakeholders, which allowed stakeholders to review and
comment on the draft data submission guide, prior to publishing the final guide. HCA has final approval authority
over the data submission guides and all subsequent changes.
For recent updates about the Drug Price Transparency (DPT) program, please see the link below:
https://www.hca.wa.gov/billers-providers-partners/prescription-drug-cost-transparency-update
Contacts
Compliance Questions or General Program Questions
For compliance questions or general questions about the Drug Price Transparency program, not related to technical
data submissions, please contact the program staff by sending an email to:
Technical Support
For technical assistance related to questions about data definitions, formatting, or the data submission process,
please contact the technical support staff by sending an email to:
HCADPTTechSuppor[email protected]v
Definitions
"Authority" means the Health Care Authority.
"Calendar days" means the same as in Washington Administrative Code 182-526-0010.
“Calendar year” means the period from January 1 to December 31 of each year.
"Covered drug" means any prescription drug that:
(a) A covered manufacturer intends to introduce to the market in Washington State at a wholesale
acquisition cost of ten thousand dollars or more for a course of treatment lasting less than one month or a
thirty-day supply, whichever period is longer; or
(b) Meets all of the following:
(i) Is currently on the market in Washington state;
(ii) Is manufactured by a covered manufacturer; and

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
4
(iii) Has a wholesale acquisition cost of more than one hundred dollars for a course of treatment
lasting less than one month or a thirty-day supply, and, taking into account only price increases that
take effect after July 28, 2019, the manufacturer increases the wholesale acquisition cost such that:
(A) The new wholesale acquisition cost is twenty percent higher than the wholesale
acquisition cost on the same day of the month, twelve months before the date of the
proposed increase; or
(B) The new wholesale acquisition cost is fifty percent higher than the wholesale acquisition
cost on the same day of the month, thirty-six months before the date of the proposed
increase.
"Covered manufacturer" means a person, corporation or other entity engaged in the manufacture of prescription
drugs sold in or into Washington state. "Covered manufacturer" does not include a private label distributor or retail
pharmacy that sells a drug under the retail pharmacy's store label, or a prescription drug repackager.
"Data" means all data provided to the authority under RCW 43.71C.020 through 43.71C.080 and any analysis
prepared by the authority.
"Data submission guide" means the document that identifies the required data to be reported under RCW 43.71C,
and provides instructions for submitting this data to the authority, including guidance on required format.
"Food and drug administration (FDA) approval date" means the deadline for the FDA to review applications for new
drugs or new biologics after the new drug application or biologic application is accepted by the FDA as complete in
accordance with the Prescription Drug User Fee Act of 1992 (106 Stat. 4491; P.L. 102-571).
"Introduced to market" means marketed in Washington State.
"Pipeline drug" means a drug or biologic product containing a new molecular entity, not yet approved by the Food
and Drug Administration, for which a manufacturer intends to seek initial approval from the Food and Drug
Administration under an original new drug application under 21 U.S.C. Sec. 355(b) or under a biologics license
application under 42 U.S.C. Sec. 262 to be marketed in Washington State.
"Prescription drug" means a drug regulated under chapter 69.41 or 69.50 RCW, including generic, brand, specialty,
and biological products that are prescribed for outpatient use and distributed in a retail setting.
"Rebate" means negotiated price concessions, discounts, however characterized, that accrue directly or indirectly to
a reporting entity in connection with utilization of prescription drugs by reporting entity members including, but is
not limited to, rebates, administrative fees, market share rebates, price protection rebates, performance-based price
concessions, volume-related rebates, other credits, and any other negotiated price concessions or discounts that are
reasonably anticipated to be passed through to a reporting entity during a coverage year, and any other form of price
concession prearranged with a covered manufacturer, dispensing pharmacy, pharmacy benefit manager, rebate
aggregator, group purchasing organization, or other party which are paid to a reporting entity and are directly
attributable to the utilization of certain drugs by reporting entity members.
"Reporting entity" means carriers, covered manufacturers, health carriers, health plans, pharmacy benefit managers,
and pharmacy services administrative organizations, which are required to or voluntarily submit data according to
chapter 43.71C RCW.
"Wholesale acquisition cost" means, with respect to a prescription drug, the manufacturer's list price for the drug to
wholesalers or direct purchasers in the United States, excluding any discounts, rebates, or reductions in price, for the
most recent month for which the information is available, as reported in wholesale acquisition cost guides or other
publications of prescription drug pricing.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
5
Submission Schedule
The table below describes the schedule of submissions that are required for you to be in compliance with the DPT
program.
Report Type
Submission Due Date
Description
New Covered
Drugs and
qualifying price
increases
(a) Sixty days in advance
of a qualifying prices
increase for a covered
drug marketed in
Washington State; or
(b) Within thirty days of
a new covered drug’s
introduction to market in
Washington State.
A covered manufacturer must submit to the authority all data
specified in RCW 43.71C.050 and 43.71C.070, following the
guidelines set forth in this data submission guide, for each newly
marketed covered drug or a covered drug that had a qualifying
price increase on or after October 16, 2020, as follows:
(a) Sixty days in advance of a qualifying prices increase for a
covered drug marketed in Washington State; or
(b) Within thirty days of a new covered drug’s introduction to
market in Washington State.
New Drug
Application
(notice from FDA
that drug will be
reviewed by
deadline)
Within sixty calendar
days of the manufacturer
receiving the FDA
approval date.
A manufacturer must submit to the authority all data specified in
RCW 43.71C.060(1), following the guidelines set in the authority’s
applicable data submission guide for all new drug applications or
biologic license applications for pipeline drugs submitted on or
after October 16, 2020, within sixty calendar days of the
manufacturer receiving the FDA approval date.
How to Register
In order to submit data to HCA, you must first complete the registration process and receive credentials for the
Secure File Transfer (SFT) service offering hosted by Washington Technology Solutions (WATECH).
To register, you must complete and submit the registration form to HCA. You can access the form at the link below.
Once you’ve completed the required information in the form, click the “Submit” button to generate an email.
Registering thirty days in advance of a reporting due date for this program is strongly encouraged, in order to ensure
ample time to be added to the system. Once your registration is processed, you will receive a user ID and password
from HCA to access the SFT service to submit data to HCA.
https://www.hca.wa.gov/assets/billers-and-providers/13-0051-drug-price-transparency-submitter-registration.pdf
Please email DrugTran[email protected]a.gov for any questions or concerns about the form and the registration
process.
How to Submit
To submit files for the Drug Price Transparency program, you will need to use the Secure File Transfer (SFT) service
offering by Washington Technology Solutions (WATECH). The SFT credentials will be provided to you by HCA. This will
allow you access to a personalized folder for your organization, where you can upload your submissions.
For more details on the process of connecting to SFT, and the tools that can be used to do so, please see “

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
6
Appendix A – ST Web Client User Guideline” and “
Appendix B – SFT Client Options (Partial List)”.
There are checks in place to protect the SFT service which may result in the rejection of your submission, without
notice. These limits include (but are not limited to) attempting to upload a file greater than 30GB and uploading or
downloading more than 50,000 files in a 24-hour period. It is unlikely that you will ever trigger these protections, as
the size and frequency of the submissions required for this program will seldom approach these limits. However,
accidentally exceeding them could result in termination of your SFT credentials. If you suspect that your SFT
credentials are no longer working, please contact the DPT program staff.
Submission Specifications
Data Validation
Data validation is a two-step process and at any time submissions may be rejected. If rejected, reports need to be
resubmitted within 10 days.
Step 1 Technical validation - If your submission passes, you will receive a confirmation email at the registered email
address for your organization. If your submission is rejected, you will receive an email with an error log
attached describing why your file was rejected. If you do not receive an email notification of either success or
failure within 72 hours of submitting your report, please contact DPT program staff at
[email protected].gov to confirm that your submission was received and processed.
Step 2 Program validation – An analyst will validate information submitted in ensure it meets program
requirements. You will receive an approval email or a rejection email. This email will be sent to the email
provided when you registered. If your report is rejected, you will need to resubmit within 10-days.
Each submitted file undergoes technical and program validation to ensure that the data meets the requirements of
RCW 43.71C and is compatible with HCAs reporting software. The technical validation process is automated and
applied shortly after submission to ensure that the data meets all of the technical rules described in the Table
Specifications. These primarily cover verification of data types (number vs. string) and formats (2021-01-01 vs.
01/01/2021). The program validation process is performed by program staff after technical validation and includes
additional checks of the files to complete the data validation process.
If you need help understanding your error log, the Data Submission FAQ clarifies the meaning of the error and
provides guidance on corrections, or you may submit your questions to HCADPTTechSupport@hca.wa.gov for
assistance.
Resubmissions
Failed Technical or Program Validations
In the event that your submission is rejected, you have 10 days after you receive the initial rejection notice to make
necessary corrections and resubmit. You may request an extension of the due date subject to HCA approval. If you
fail to comply with reporting requirements after receiving a rejection notice, the authority may assess a fine as
allowed under WAC 182-51-1300.
To ensure that you receive credit for a resubmission, you should use the same YYYYMMDD value in the file name as
you did in your first submission.
For example, if you submitted the file ‘manufacturer_covered_drugs_2021_M12345_20210301.csv’, and
received a rejection, after making corrections you should resubmit the file
‘manufacturer_covered_drugs_2021_M12345_20210301.csv’ with the same name as it was originally
submitted under, even if the date of resubmission is a different date.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
7
Corrective Submissions
In the event that you find an error in your approved submission, you will need to fill out the Resubmission form
which can be found on our website prior to resubmitting your report. You will need to let HCA know which report
you will be resubmitting and the specific reasons why you request to resubmit. HCA will review your request and
approve or deny your request within 5 business days. In the event your resubmission is rejected during validation,
you would be subject to the 10 day limit for correcting rejected resubmissions.
File Specifications
All files submitted must be text files with comma-separated values (CSV). The text should be encoded using the UTF-8
standard. Line endings in UNIX (“\n”) or Windows (“\r\n”) format are both acceptable. The header row must be
included in every file. For detailed technical guidance, see the Library of Congress CSV Definition.
Appropriately formatted files can also be generated via Microsoft Excel by saving a spreadsheet in CSV format. This
will remove many of the features included in Excel, such as formatting, formulas, and “sheets”, so you may want to
save a copy in Excel format for your own reference in the future. We recommend using Microsoft Excel 2016 or
earlier for the submission guide templates. Using Microsoft Excel 2019 or Microsoft 365 can cause formatting issues
when saving as a CSV file and result in errors.
File names should follow the naming scheme specified for the specific data that you are submitting. See Table
Specifications section for more information.
Data Specifications
Nullable: All fields are required, unless otherwise indicated in the table specification. A field that is not required, will
be indicated with the word “Nullable” in the specification. In those cases, you must leave that field blank. Do NOT
provide the value as “NULL”, or otherwise provide a special indicator of a null value. In all other cases, providing a
blank value will result in a rejection by the automated validation.
Date Formats: Unless otherwise specified, all dates should be reported in ISO-8601 format with hyphens between
years, months, and days: “YYYY-MM-DD”. For example, December 1, 2022, would be recorded as “2022-12-01”.
Important note about Excel version: We recommend using Microsoft Excel 2016 or earlier for the submission guide
templates. Using Microsoft Excel 2019 can cause formatting issues when saving as a CSV file and result in the file
being rejected.
Table Specifications
New Covered Drugs and Qualifying Price Increases
This report contains all of the fields necessary to comply with the notification of a price increase and covered drug as
described in RCW 43.71C.050 and 43.71C.070. Files submitted for manufacturer covered drugs should be named
using the following schema, where ID is the manufacturer ID assigned to you by HCA during the registration process
(Washington DPT Number), YYYY is the current calendar year, and YYYYMMDD is a placeholder for the submission
date. In the case of a resubmission after file rejection, please use the same value for YYYYMMDD as the file that was
rejected. Do not replace “manufacturer” with your organizations name, this will result in your submission being
rejected.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
8
File naming schema: manufacturer_covered_drugs_{YYYY}_{ID}_{YYYYMMDD}.csv
Example: manufacturer_covered_drugs_2022_M12345_20210301.csv (Please use the
submission due date, not the date the report was prepared)
For a price increase that occurred prior to the current calendar year the YYYY should be populated with the year the
price increase took effect.
For example:
manufacturer_covered_drugs_2019__M12345_20210301.csv or
manufacturer_covered_drugs_2020__M12345_20210301.csv or
manufacturer_covered_drugs_2021__M12345_20210301.csv
Please see the Submission Schedule for details regarding the timelines for submitting reports for covered drugs.
Specification
Description
Name: Washington DPT Number
Type: String
Max Length: 6 characters
Format: ABCDE
WA Drug Price Transparency (DPT) assigned unique submitter identifier upon
registration with the Health Care Authority Drug Price Transparency program.
This number is unique to you and follows a format of either CXXXXX, MXXXXX,
SXXXXX or PXXXXX where C, M, S and P indicate whether you are a carrier,
manufacturer, PSAO or PBM. The X’s are numeric digits e.g. 12345.
Name: Manufacturer Name
Type: String
Max Length: 80 characters
Format: ABCDE
Labeler name of entity who markets the drug. This entity has the corresponding
Labeler Code in the following data field.
Name: Labeler Code
Type: Numeric
Format: 00000
Max Length: 5 digits
Labeler code as assigned by Food and Drug Administration (FDA) These 5 digits
should match the first 5 digits of all submitted NDCs in this report.
Name: NDC
Type: Numeric
Format: 00000000000
Max Length: 11 digits
Min Length: 11 digits
A three-segment code maintained by the Federal Food and Drug Administration
that includes a labeler code, a product code, and a package code for a drug
product (e.g., 12345678910)
NOTE: The NDC field must be eleven digits long and maintain leading
zeros.
Name: Drug Name
Type: String
Max Length: 100 characters
Format: ABCDE
Name of the drug for the NDC reported. Only include ingredient name.
For example, if the NDC has a Drug Product Name of "fluoxetine HCL 20
mg tablets", then this field should be reported as “fluoxetine”. All drug
product names with “fluoxetine” in its name should be reported as a
single Drug Name in this field. Combination drug product names should
be reported individually as its own Drug Name instead of by each
ingredient.
NOTE: Special characters, hyphens, symbols, or slashes are allowed.
Name: Drug Product Name
Type: String
Max Length: 100 characters
Format: ABCDE
Name of the drug product for the NDC reported, to include ingredient
name as reported in standardized drug databases. This name should
include ingredient, salt form, dosage form, strength, and any other
information specific to the NDC.
For example, "fluoxetine HCL 20 mg tablets" is acceptable.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
9
Name: Label Name
Type: String
Max Length: 100 characters
Format: ABCDE
Proprietary or legal name as marketed by manufacturer. For example,
"fluoxetine HCL", "fluoxetine DR” are acceptable.
Name: Drug Type
Type: Choice
Choices: S, N, I
Drug Type is one of following values:
Single Source (S) – Drugs with an FDA New Drug Application (NDA), or
biologics with a Biologics License Application (BLA), and for drugs, there
are no generic alternatives available on the market.
Non-Innovator Multiple-Source (N) – Drugs with an FDA Abbreviated
New Drug Application (ANDA).
Innovator Multiple-Source (I) – Drugs with an NDA and no longer have
patent exclusivity.
Name: Unit of Measure
Type: Choice
Choices: AHF, CAP, SUP, GM, ML, TAB,
TDP, EA
Unit of Measure for Wholesale Acquisition Cost (WAC) defined as one
of the following values:
AHF: Anti-hemophilia factor
CAP: Capsule
SUP: Suppository
GM: Gram
ML: Milliliter
TAB: Tablet
TDP: Transdermal patch
EA: Each
Name: Day Supply
Type: Numeric
Format: 000
Max Length: 3 digits
Min Length: 1 digit
Indicate estimated day supply in relation to package size.
Example: Package size of 100 used once daily will equal a 100.
Package supply of 100 used 5 x’s a day will equal a 20.
Name: Package Size
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
The package size identifies the number of billing units (as specified
by the labeled quantity) in the package the pharmacist uses to
dispense; for example, 100 tablets, 1000 capsules, or 20 ml vial. The
package quantity complies with the National Council of Prescription
Drug Programs (NCPDP) Billing Unit Standard.
Name: Qualifying Price Increase
Type: Choice
Choices: Y, N
Indicator for qualifying price increase. Manufacturer must use this field
as 'yes' or 'no' to indicate if the drug meets the criteria of a qualifying
price increase as defined in RCW 43.71C.010(8).
Name: WAC – Current (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Nullable
The wholesale acquisition cost per unit of measure on the date of the
submission (60 days prior to the effective date of the WAC increase). If
the covered drug report is for a drug being introduced to the market,
then leave blank.
NOTE: Do not include the dollar sign or commas.
Name: WAC – Current (Package Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Nullable
The wholesale acquisition cost per package on the date of the
submission (60 days prior to the effective date of the WAC increase). If
the covered drug report is for a drug being introduced to the market,
then leave blank.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
10
Name: WAC Effective Date
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Effective date of the wholesale acquisition cost increase for the drug
product. If the covered drug report is for a new covered drug being
introduced to the market, then this field should be the date the product
will first be available.
Name: WAC Type
Type: Choice
Choices: Package, Unit or Both
Manufacturer must indicate if reporting by package, unit price or both.
Package – Complete WAC Increase (Package Price) and WAC – New
(Package Price) fields.
Unit – Complete WAC Increase (Unit Price) and WAC – New (Unit Price)
fields.
Both – Complete WAC Increase (Package Price), WAC Increase (Unit
Price), WAC - New (Package Price) and WAC – New (Unit Price).
Name: WAC Increase (Unit Price)
Type: Numeric
Format: 999999.99999
Max Length: 11 digits
Rule: Required when “WAC Type” field is
“Unit” or “Both”, Nullable if WAC Type =
“Package” or Nullable if new drug
introduced to market
Amount of wholesale acquisition cost increase per unit of measure for
the drug product. Express this as a dollar amount up to 5 decimal
places. If the covered drug report is for a new drug being introduced to
the market, leave blank.
NOTE: Do not include the dollar sign or commas.
Name: WAC Increase (Package Price)
Type: Numeric
Format: 999999.99999
Max Length: 11 digits
Rule: required if “WAC Type” field is
“Package” or “Both”
Nullable if WAC Type = “Unit” or
Nullable if new drug introduced to
market
Amount of wholesale acquisition cost increase per package for the drug
product. Express this as a dollar amount up to 5 decimal places. If the
covered drug report is for a new drug being introduced to the market,
leave blank.
NOTE: Do not include the dollar sign or commas.
Name: WAC – New (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Required when “WAC Type” field
is “Unit” or “Both”
Nullable if WAC Type = “Package”
The new wholesale acquisition cost (WAC) per unit of measure on the
WAC effective date. If the covered drug report is for a new covered drug
being introduced to the market, then this field should be the WAC on
the date the product is first available.
NOTE: Do not include the dollar sign or commas.
Name: WAC – New (Package Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Required when “WAC Type” field
is “Package” or “Both”
Nullable if WAC Type = “Unit”
The new wholesale acquisition cost (WAC) per package on the WAC
effective date. If the covered drug report is for a new covered drug
being introduced to the market, then this field should be the WAC on
the date the product is first available.
NOTE: Do not include the dollar sign or commas.
Name: Existing Manufacturer Drug
Type: Choice
Choices: Y, N
Mark “Y” if the drug has been manufactured by the manufacturer for the
previous 5 years. If “Y”, the WAC for the previous 5 years must be
reported.
Mark “N” if the drug has been manufactured by the manufacturer for
less than 5 years. The WAC for the previous 5 years is not required.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
11
Name: WAC - 1 Year Prior (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and Rule: Required when “WAC Type”
field is “Unit” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Package”
Wholesale acquisition cost per unit of measure 12 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 2 Year Prior (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Unit” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Package”
Wholesale acquisition cost per unit of measure 24 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 3 Year Prior (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Unit” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Package”
Wholesale acquisition cost per unit of measure 36 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 4 Year Prior (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule Required when “WAC Type” field
is “Unit” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Package””.
Wholesale acquisition cost per unit of measure 48 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 5 Year Prior (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Unit” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Package”
Wholesale acquisition cost per unit of measure 60 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
12
Name: WAC - 1 Year Prior (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Package” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Unit”
Wholesale acquisition cost per package 12 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 2 Year Prior (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Package” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Unit”
Wholesale acquisition cost per package 24 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 3 Year Prior (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Package” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Unit”
Wholesale acquisition cost per package 36 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: WAC - 4 Year Prior (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Package” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Unit”
Wholesale acquisition cost per package 48 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
13
Name: WAC - 5 Year Prior (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: Existing Manufacturer Drug “Y”,
and
Rule: Required when “WAC Type” field
is “Package” or “Both”, value must be
greater than zero,
Nullable if WAC Type = “Unit”
Wholesale acquisition cost per package 60 months prior to WAC
Effective Date.
This field must be populated if you have manufactured this drug for 5 or
more years.
NOTE: Do not include the dollar sign or commas.
Name: Change/Improvement
Description
Type: String
Max Length: 5000 characters
Format: ABCDE
Rule: value is populated when column
"Qualifying Price Increase" is equal to Y
A narrative description of any change or improvement in the drug that
necessitates the WAC increase.
Name: Financial Factors
Type: String
Max Length: 5000 characters
Format: ABCDE
A narrative description of the specific financial factors used to make the
decision to set the WAC for a new Covered Drug or to increase the
wholesale acquisition cost of an existing Covered Drug.
Name: Non-financial factors
Type: String
Max Length: 5000 characters
Format: ABCDE
A narrative description of the specific non-financial used to make the
decision to set the WAC for a new Covered Drug or to increase the
wholesale acquisition cost of an existing Covered.
Name: Patent Expiration Date
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Rule: Must be populated if “Drug Type
= S.
The date when all patents on the drug product will expire. Patents
owned by the manufacturer (i.e., originator or the inventor). Blanks are
acceptable if the drug type field is "N“or “I”.
Name: Market Entry Date
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
The date the drug was Introduced to Market in Washington state.
Name: WAC Market Entry Type
Type: Choice
Choice: Package, Unit or Both
Manufacturer must indicate if reporting by package, unit price or both.
Name: WAC – Unit Market Entry
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Market Entry Date" is populated, and
WAC Market Entry Type indicates
“Unit” or “Both”
Nullable if WAC Market Entry =
“Package”
The wholesale acquisition cost per unit of measure for the existing
Covered Drug on the Market Entry Date of that Covered Drug.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
14
Name: WAC – Package Market Entry
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Market Entry Date" is populated, and
WAC Market Entry Type indicates
“Package” or “Both”
Nullable if WAC Market Entry = “Unit”
The wholesale acquisition cost per package for the existing Covered Drug
on the Market Entry Date of that Covered Drug.
NOTE: Do not include the dollar sign or commas.
Name: Reporting Period From
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Nullable for new drug introduced to
market
The starting date of the period being used to report for annual
manufacturing, marketing, and advertising costs. Report the most
recent completed calendar year.
For example, if the effective date of the WAC increase is January 1, 2022,
through February 28, 2022, report calendar year 2020. If the effective
date of the WAC increase is March 1, 2022, through December 31, 2022,
report calendar year 2021.
Name: Reporting Period To
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Nullable for new drug introduced to
market
The ending date of the period being used to report for annual
manufacturing, marketing, and advertising costs. Report the most recent
completed calendar year.
For example, if the effective date of the WAC increase is January 1, 2022,
through February 28, 2022, report calendar year 2020. If the effective
date of the WAC increase is March 1, 2022, through December 31, 2022,
report calendar year 2021.
Name: Manufacturing Costs
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
The total cost to produce the number of units manufactured in most
recent completed calendar year prior to the WAC Effective Date.
NOTE: Do not include the dollar sign or commas.
Name: Marketing and Advertising
Costs
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Nullable
Amount spent on marketing and advertising, in the most recent
completed calendar year prior to the WAC Effective Date, including but
not limited to direct-to-consumer marketing (television, radio print,
digital, etc.), salaries for sales representatives, salaries for medical
liaisons, hosted CE events and provider education, and provider
detailing.
NOTE: Do not include the dollar sign or commas.
Name: Clinical Trials Costs
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Total costs for all clinical trials for the covered drug.
NOTE: Do not include the dollar sign or commas.
Name: Research and Development
Cost
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Total expenditure on research and development prior to Market Entry
Date.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
15
Name: Regulation Costs
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
All costs paid by the manufacturer to the FDA and any other regulatory
body for considering their drug application and bringing the drug to
market.
NOTE: Do not include the dollar sign or commas.
Name: Acquired from Previous
Manufacturer
Type: Choice
Choices: Y, N
Indicator for whether the drug was acquired from another manufacturer
in the previous 5 years. Manufacturer must use this field as 'yes' or 'no'
to indicate if the drug meets the criteria in RCW 43.71C.050(4)?
Name: Previous Owner's Name
Type: String
Max Length: 80 characters
Format: ABCDE
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
The legal name of entity who sold the covered drug to the manufacturer.
Name: Previous Manufacturer ID
Type: Numeric
Format: 00000
Max Length: 5 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
If the drug product was purchased from another manufacturer,
repackager, or private label distributor, the labeler code as assigned by
Food and Drug Administration (FDA). If previous owner does not have a
labeler ID fill with 5 zeros.
Name: Previous NDC
Type: Numeric
Format: 00000000000
Max Length: 11 digits
Min Length: 11 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
The NDC that was used by the original or previous manufacturer. For
new drug products that do not have a previous NDC fill with eleven
zeros.
NOTE: The NDC field must be eleven digits long and maintain leading
zeros.
Name: Purchase Price
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
If the drug product was not developed by the manufacturer, the amount
the manufacturer paid to acquire the drug.
NOTE: Do not include the dollar sign or commas.
Name: Currency of Purchase
Type: String
Max Length: 50 characters
Format: ABCDE
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
The country of acquisition and type currency used to acquire the drug
e.g., USD, EUR, GBP, CAD, JPY, AUD, INR, CNY, MXN, etc.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
16
Name: Acquisition Date
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y
Nullable
If the drug product was not developed by the manufacturer, the date the
manufacturer acquired the drug.
Name: WAC - Acquisition Type
Type: Choice
Choice: Package, Unit or Both
Manufacturer must indicate if reporting by package, unit, or both.
Name: WAC – Acquisition (Unit Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y and WAC
Acquisition Type indicates “Unit” or
“Both”
Nullable if WAC Acquisition Type =
“Package”
The wholesale acquisition cost per unit of measure for the drug product
on the acquisition date.
NOTE: Do not include the dollar sign or commas.
Name: WAC – Acquisition (Package
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y and WAC
Acquisition Type indicates “Package” or
“Both”
Nullable if WAC Acquisition Type =
“Unit”
The wholesale acquisition cost per package for the drug product on the
acquisition date.
NOTE: Do not include the dollar sign or commas.
Name: WAC - Prior to Acquisition Type
Type: Choice
Choice: Package, Unit or Both
Manufacturer must indicate if reporting by package, unit, or both.
Name: WAC - Prior to Acquisition (Unit
Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y and WAC
Prior to Acquisition Type indicates
“Unit” or “Both”
Nullable if WAC Prior to Acquisition
Type = “Package”
The wholesale acquisition cost per unit of measure for the drug product
12 months prior to the acquisition date.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
17
Name: Unit of Measure – Prior to
Acquisition
Type: Choice
Choices: AHF, CAP, SUP, GM, ML, TAB,
TDP, EA
Rule: value is populated when column
“WAC – Prior to Acquisition” is equal to
any non-zero value
Nullable
Unit of Measure for WAC (prior to acquisition) defined as one of the
following values:
AHF: Anti-hemophilia factor
CAP: Capsule
SUP: Suppository
GM: Gram
ML: Milliliter
TAB: Tablet
TDP: Transdermal patch
EA: Each
Name: WAC - Prior to Acquisition
(Package Price)
Type: Numeric
Format: 999999999.99999
Max Length: 14 digits
Rule: value is populated when column
"Acquired from Previous
Manufacturer" is equal to Y and WAC
Prior to Acquisition Type indicates
“Package” or “Both”
Nullable if WAC Prior to Acquisition
Type? = “Unit”
The wholesale acquisition cost per package for the drug product 12
months prior to the acquisition date.
NOTE: Do not include the dollar sign or commas.
Name: Financial Assistance Program
Costs
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Rule: greater than or equal to 0
Total cost of all financial assistance programs including financial
assistance for uninsured individuals, compassionate use, patient
assistance, charity care, donated drug product, etc., associated with the
drug product for the calendar year prior to the WAC Effective Date. For
example, if the WAC Effective Date is March 1, 2020, report the total
amount spent on financial assistance programs in calendar year 2019. If
no financial assistance was provided fill with zeros.
NOTE: Do not include the dollar sign or commas.
Name: Rebates
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Rule: greater than or equal to 0
Total amount of rebates paid out associated with the NDC in the
calendar year prior to the WAC Effective Date.
For example, if the effective date of the WAC increase is between and
including January 1, 2022, through February 28, 2022, report calendar
year 2020.If the WAC Effective Date is March 1, 2022, report the total
amount of rebates paid to any entity in calendar year 2021. If no rebates
were provided fill with zeros.
NOTE: Do not include the dollar sign or commas.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
18
Name: Cost Share Assistance
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Rule: greater than or equal to 0
Total amount of money paid toward lowering an insured individual's out
of pocket expenditure for the drug product in the calendar year prior to
the WAC Effective Date.
For example, if the effective date of the WAC increase is between and
including January 1, 2022, through February 28, 2022, report calendar
year 2020. If the WAC Effective Date is March 1, 2022, report the total
amount spent on cost share assistance in calendar year 2021. If no
financial assistance was provided fill with zeros.
NOTE: Do not include the dollar sign or commas.
Name: Other Financial Assistance
Amount
Type: Numeric
Format: 999999999999999.99
Max Length: 17 digits
Rule: greater than or equal to 0
Total amount of all other financial assistance paid out associated with
the NDC in the calendar year prior to the WAC Effective Date.
For example, if the effective date of the WAC increase is between and
including January 1, 2022, through February 28, 2022, report calendar
year 2020. I the WAC Effective Date is March 1, 2022, report the total
amount of all other financial assistance paid to any entity in calendar
year 2021. If no other financial assistance was provided fill with zeros.
NOTE: Do not include the dollar sign or commas.
Name: General Comments
Type: String
Format: ABCDE
Max Length: 5000 characters
Nullable
Any additional information you would like to submit or provide to
explain your responses.
New Drug Application
This report contains all of the data fields necessary to comply with reporting a New Drug Application to HCA, per
RCW 43.71C.060.
Files submitted for manufacturer new drugs should be named using the following schema, where: ID is the
manufacturer ID assigned to you by HCA during the registration process (Washington DPT Number), YYYY is the
current reporting period, and YYYYMMDD is a placeholder for the submission date. In the case of a resubmission
after file rejection, please use the same value for YYYYMMDD as the file that was rejected. Do not replace
“manufacturer” with your organizations name, this will result in your submission being rejected.
File naming schema: manufacturer_new_drugs_{YYYY}_{ID}_{YYYYMMDD}.csv
Example: manufacturer_new_drugs_2022__M12345_20210301.csv (Please use the submission
due date, not the date the report was prepared)
For a new drug application that occurred prior to the current calendar year the YYYY should be populated with the
year the price increase took effect.
For example:
manufacturer_new_drugs_2019__M12345_20210301.csv or

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
20
Specification
Description
Name: Washington DPT Number
Type: String
Max Length: 6 characters
Format: ABCDE
WA Drug Price Transparency (DPT) assigned unique submitter identifier
upon registration with the Health Care Authority Drug Price
Transparency program.
This number is unique to you and follows a format of either CXXXXX,
MXXXXX, SXXXXX or PXXXXX where C, M, S and P indicate whether you
are a carrier, manufacturer, PSAO or PBM. The X’s are numeric digits
e.g. 12345.
Name: Manufacturer Name
Type: String
Max Length: 80 characters
Format: ABCDE
Labeler name of entity who manufactures and markets the drug.
Name: Labeler Code
Type: Numeric
Format: 00000
Max Length: 5 digits
Labeler code as assigned by Food and Drug Administration (FDA)
Name: Drug Name
Type: String
Max Length: 80 characters
Format: ABCDE
Name of the drug for the NDC reported. Only include ingredient name.
For example, if the NDC has a Drug Product Name of "fluoxetine HCL 20
mg tablets", then this field should be reported as “fluoxetine”. All drug
product names with “fluoxetine” in its name should be reported as a
single Drug Name in this field. Combination drug product names should
be reported individually as its own Drug Name instead of by each
ingredient.
NOTE: Special characters, hyphens, symbols, or slashes are allowed.
Name: Drug Product Name
Type: String
Max Length: 100 characters
Format: ABCDE
Nullable
Name of the drug product for the NDC reported, to include ingredient
name as reported in standardized drug databases. This name should
include ingredient, salt form, dosage form, strength, and any other
information specific to the NDC.
For example, "fluoxetine HCL 20 mg tablets" is acceptable.
Name: Label Name or Pipeline Drug
Name
Type: String
Max Length: 100 characters
Format: ABCDE
Nullable
Proprietary or legal name as marketed by manufacturer. For example,
"fluoxetine HCL", "fluoxetine DR” are acceptable.
If not approved by the FDA, then enter the name of the Pipeline Drug.
For example, “AAA600”.
Name: Drug Type
Type: Choice
Choices: S, N, I
Drug Type is one of following values:
Single Source (S) – Drugs that having an FDA New Drug Application
(NDA), or biologics having a Biologics License Application (BLA), and
there are no generic alternatives available on the market.
Non-Innovator Multiple-Source (N) – Drugs that have an FDA
Abbreviated New Drug Application (ANDA).
Innovator Multiple-Source (I) – Drugs that have an NDA and no longer
have patent exclusivity.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
21
Name: Application Type
Type: Choice
Choices: BLA, NDA, ANDA
Application Type is one of following values:
New Drug Application (NDA) – Drug is a pipeline drug and was
submitted as a New Drug Application to the FDA.
Biologics License Application (BLA) – Drugs is a pipeline drug and was
submitted as a Biologics License Application to the FDA.
Abbreviate New Drug Application (ANDA) – contains data which is
submitted to FDA for the review and potential approval of a generic
drug.
Name: Regulatory Pathway
Type: Choice
Choices: 505(b)(1), 351(a), Other
Regulatory pathway for approval by the Food and Drug Administration.
Acceptable values are 505(b)(1), 351(a) or Other.
If choosing “Other” please list the regulatory pathway this product was
approved in General Comments.
Name: Application Number
Type: Numeric
Format: 000000
Max Length: 6 digits
Min Length: 6 digits
Nullable
The application number assigned by the Food and Drug Administration.
For application numbers less than 6 digits, the application number
should be preceded using zeros.
Name: Application Supplement
Number
Type: Numeric
Format: 0000
Max Length: 4 digits
Min Length: 4 digits
Nullable
The supplemental application number assigned by the Food and Drug
Administration. For application numbers less than 4 digits, the
supplemental application number should be preceded using zeros.
Name: Significant Impact on State
Expenditures
Type: Choice
Choices: Y, N
Indicator of whether the pipeline drug will cost Washington State
government agencies at least $50,000 per biennium in any future
biennium. HCA believes that drugs costing at least $50,000 per
biennium for Washington State government agencies to qualify as a
significant impact on state expenditures. HCA may request from the
manufacturer the information in the remaining fields if HCA believes
the drug will have a significant impact on state expenditures and
require manufacturers to resubmit with information for all of the
following fields. If manufacturers believe drugs to meet or exceed this
threshold, the following fields may be completed. WAC 182-51-0700(3)
Name: Proposed Indication
Type: String
Max Length: 5000 characters
Format: ABCDE
Nullable
The proposed indication or indications submitted on the application to
the FDA. Use the SNOMED CT disease term listed on the application.
Use a semi-colon to separate multiple indications.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
22
Name: Area of Study
Type: String
Max Length: 5000 characters
Format: ABCDE
Nullable
A list of diseases, conditions, and therapeutic areas being studied for
this drug and whether the chemical drug has received an indication in
the FDA approved labeling for use in these diseases, conditions, or
therapeutic areas.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Route of Administration
Type: String
Max Length: 5000 characters
Format: ABCDE
Nullable
List each route of administration being studied for this drug, including
any differences between immediate-release and extended-release
formulations.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Clinical Comparator
Type: String
Max Length: 5000 characters
Format: ABCDE
Nullable
All clinical comparators including dosage regimen being used for which
to evaluate the comparative differences in safety, efficacy,
effectiveness, costs, value, or any other outcomes in clinical trials.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: PDUFA Date
Type: Date
Format: YYYY-MM-DD
Min Year: 1900
Max Year: 2100
Nullable
Prescription Drug User Fee Act (PDUFA) date assigned by the FDA.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Rare Disease Indication
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as being defined as a
treatment for a rare disease.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Orphan Drug Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having an Orphan
designation.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Orphan Designation Number
Type: Numeric
Format: 000000
Max Length: 6 digits
Min Length: 6 digits
Nullable
Orphan designation number assigned by the FDA. For Orphan
Designation numbers less than 6 digits, the supplemental application
number should be preceded using zeros.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
23
Name: Pediatric Indication
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the indication is for use in individuals under 18
years of age.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Fast Track Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having fast track
status.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Breakthrough Therapy Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having breakthrough
therapy status.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Accelerated Approval Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having accelerated
approval status.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: Priority Review Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having priority
review status.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: New Molecular Entity Status
Type: Choice
Choices: Y, N
Nullable
Indicator of whether the FDA assigned the drug as having new
molecular entity status.
Manufacturers may submit this information voluntarily if the pipeline
drug is expected to cost Washington State at least $50,000 per
biennium WAC 182-51-0700(3).
Name: General Comments
Type: String
Format: ABCDE
Max Length: 5000 characters
Nullable
Any additional information you would like to submit or provide to
explain your responses.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
24
Appendix A – ST Web Client User Guideline
Prerequisites
Before you can log in to ST Web Client and open a session, you need:
• A high-speed Internet connection
• A supported Internet browser:
o Microsoft Internet Explorer 11
o Microsoft Edge - latest version
o Mozilla Firefox - latest version
o Apple Safari - latest version
o Google Chrome - latest version
• A connection URL to paste into your browser: https://sft.wa.gov or https://sft-test.wa.gov
• A username and password. This information is provided to you by State of Washington business partner. You
must enter this information on the Log in page.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
25
Sign in with your password
To sign into ST Web Client:
1. Open a supported browser. Use this URL for Production Site - https://sft.wa.gov
2. Enter the connection URL and press enter. This Sign in page should be displayed.
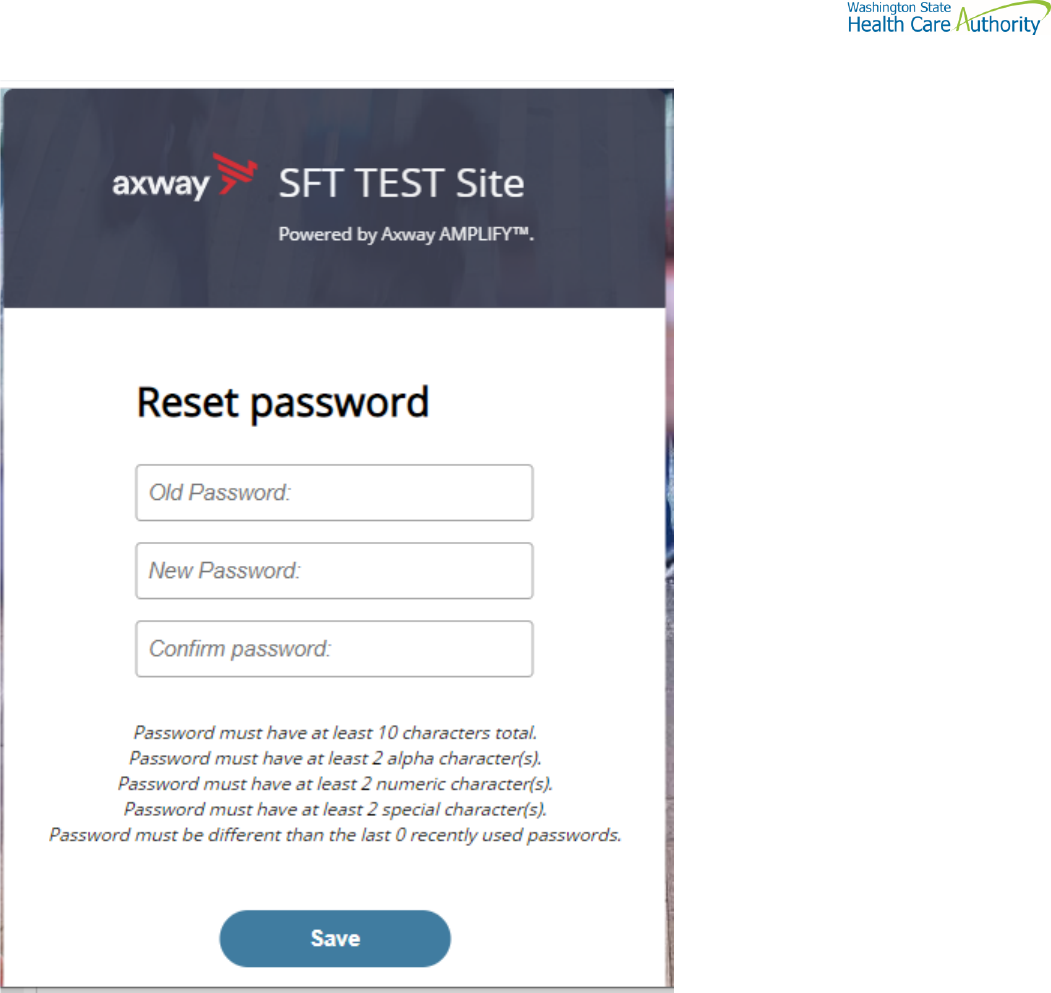
Upon signing in you may be requested to reset your password.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
26
This required when a temporary password was given to you.
Change password page is displayed as above.
If you attempt to sign in and you receive a message that indicates you must reset your password, follow these steps:
1. Enter your old password or the temporary password provided by the system administrator.
2. Enter your new password. Your new password must meet the listed criteria defined by Office of Cyber
Security State of Washington.
3. Confirm your new password.
4. Click Save.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
27
Main page in ST Web Client
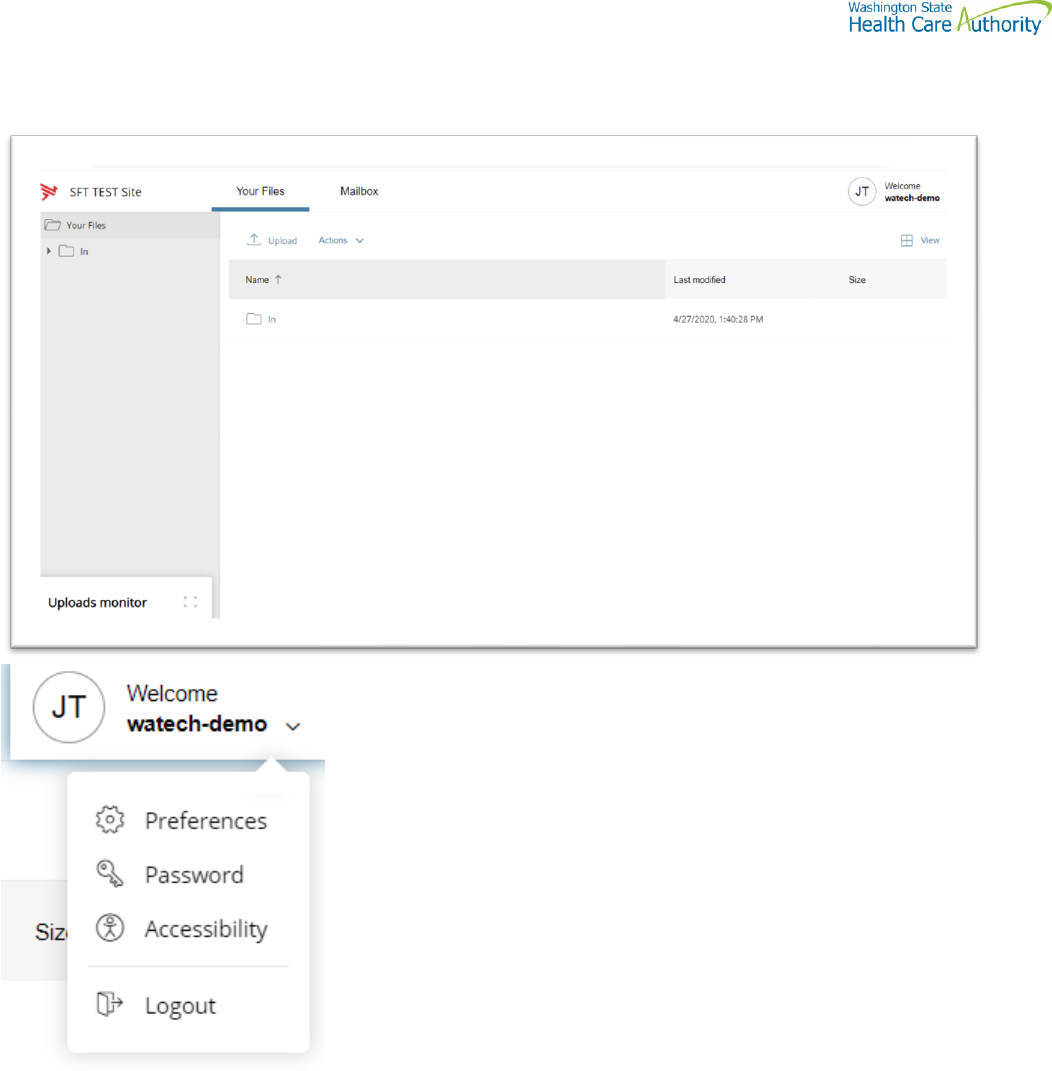
This page is displayed after successful login.
Welcome menu
Using the Welcome menu (drop down menu on the upper right corner of page), you can access the tools to manage
your user profile as well as logout.
• Log out
• Select the Welcome drop-down.
• Click Logout.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
28
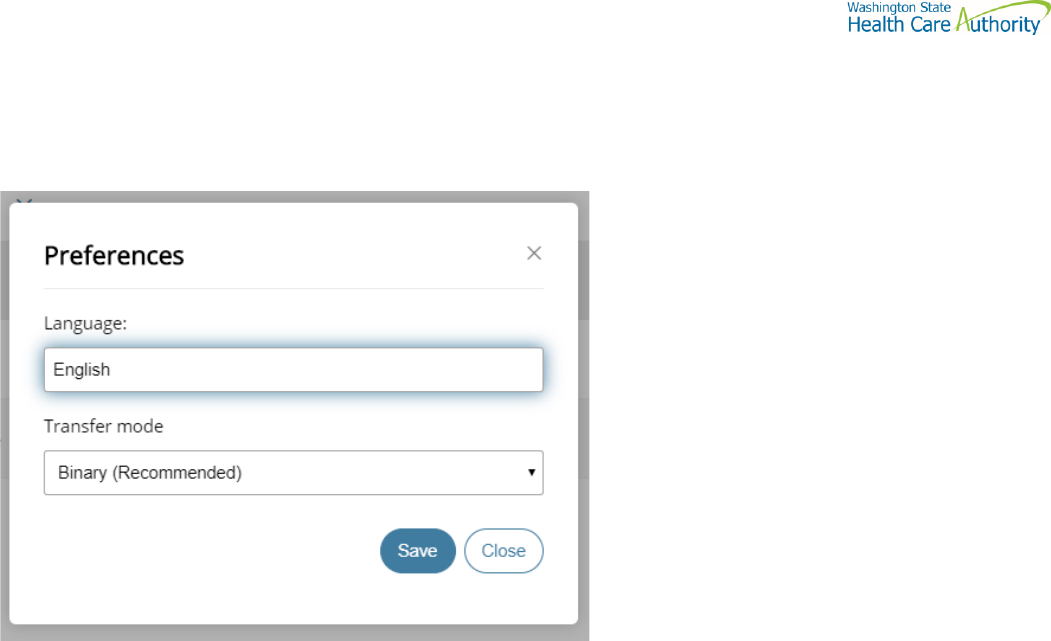
Set preferences
To set a preference:
• Select the Welcome drop-down.
• Click Preferences. The Preferences pane is displayed.
Select a Transfer mode
The recommended and default Transfer mode is
Binary
but in rare cases the
ASCII
mode may be required for XML, HTML, or TXT files.
Click Save.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
29
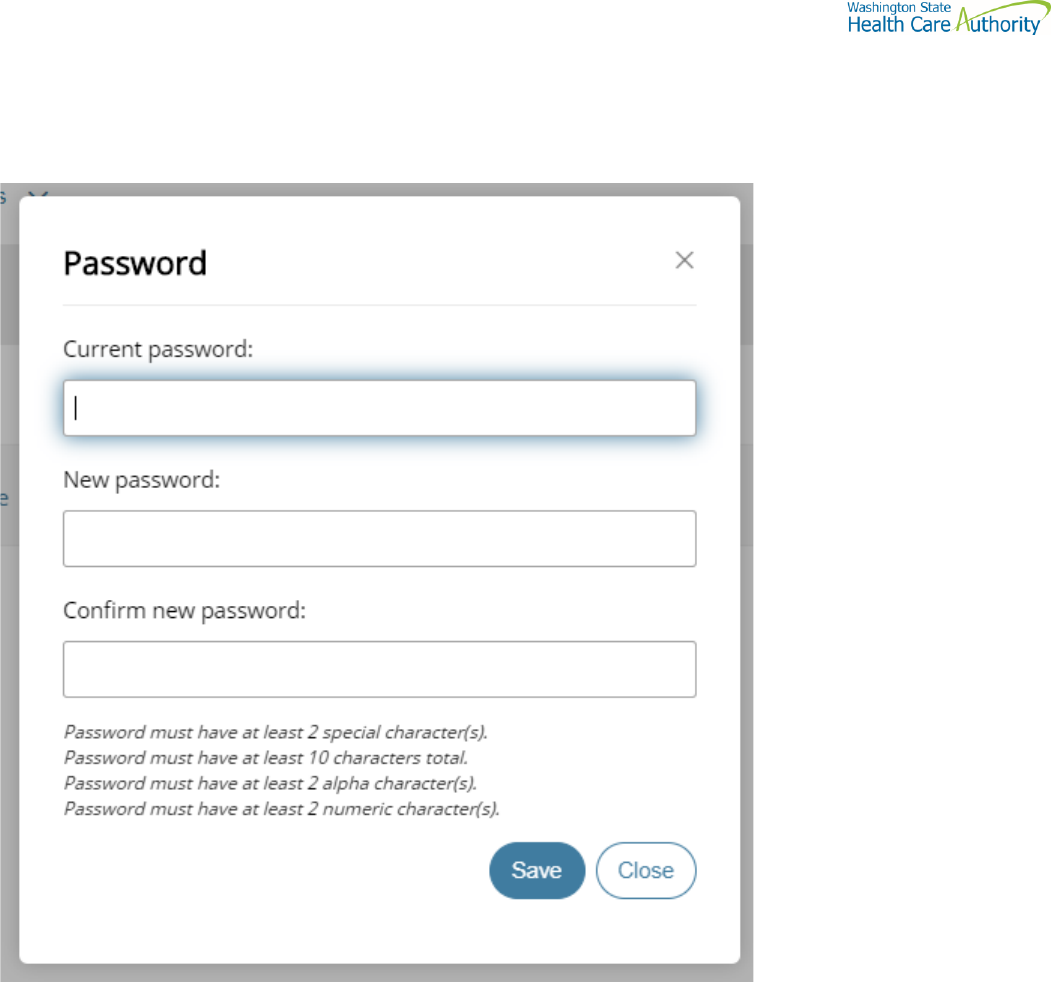
Change your password
Select the Welcome drop-down.
Click Password. The Password pane opens.
1. Enter your Current password.
2. Enter your new password.
3. Confirm new password.
4. Click Save.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
30
Upload files
To upload files to ST Web Client you click the Upload button.
From your files pane, click Upload.
Select the file or files to upload. Use the Ctrl or Shift keys to select multiple files.
Click Open.
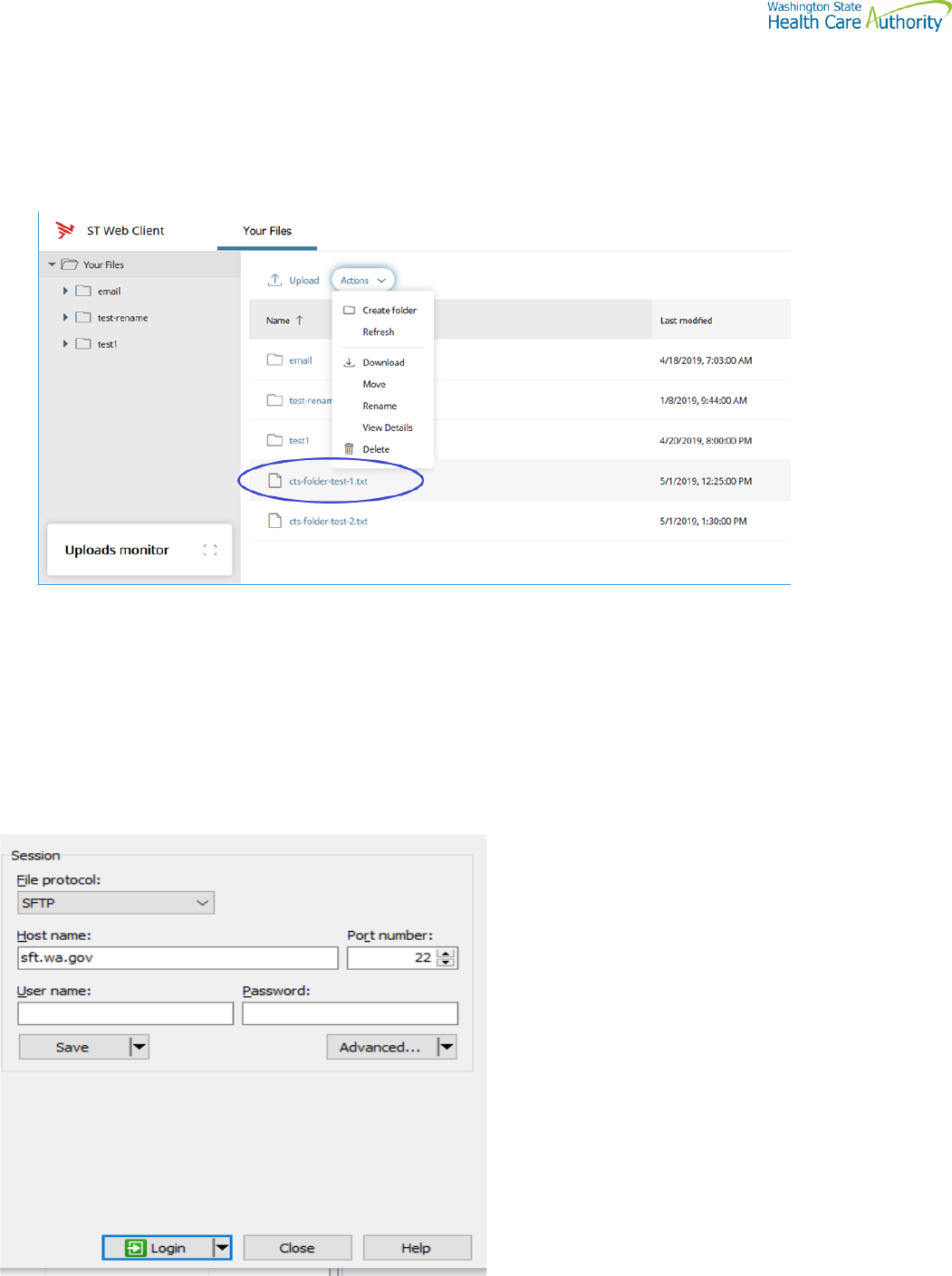
The below will be display showing progress of your file upload.
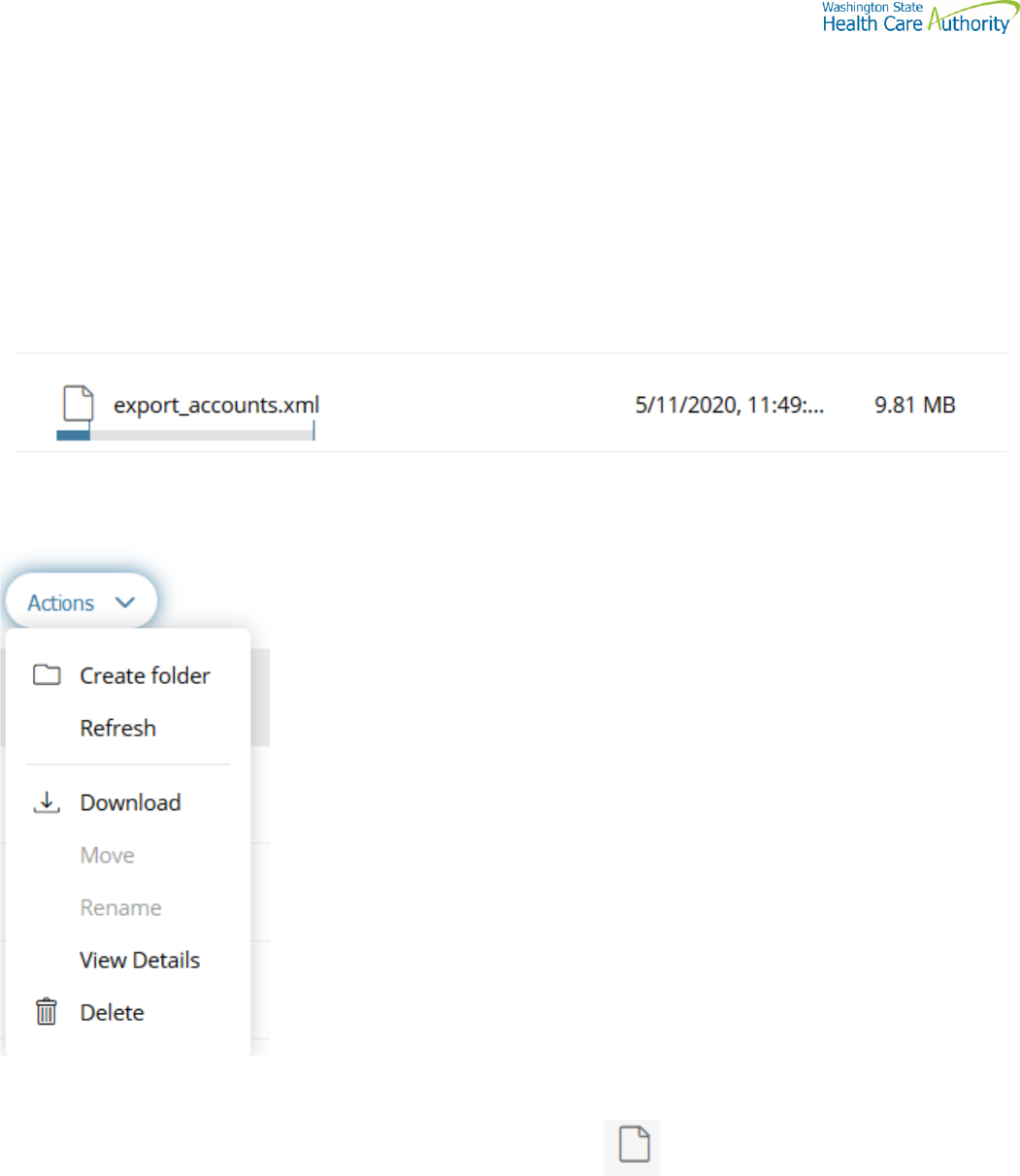
Actions Drop Down Menu
Download files
To download files from ST Web Client you click to the left of this icon on your files pane. Use the Ctrl or Shift
keys to select multiple files.
Click Action dropdown and select Download.
A popup will ask you to “Open” or “Save File”. Note: Ensure data accuracy and completeness of data download utilize
the “Save File” choice.
Create folders

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
31
To create folders
Select Create folder from the Actions Drop Down.
The Create folder pane opens.
Enter the folder name.
Click Create. The new folder is created and displayed on the “Your Files” pane and a message is displayed.
Delete files and folders
To delete a file or folder:
From the “Your Files” pane, select the file or folder to delete. Use the Ctrl key to select multiple files.
Select Delete from the Actions Drop Down menu. The delete confirmation pane opens.
Click Delete to confirm.
View file or folder details
You can view the following details of files and folders:
For files, the View Details pane lists Modified, Size, and Owner details.
For folders, the View Details pane lists Modified and Owner details.
To view file or folder details
From the “Your Files” pane, select a file or folder.
Select View Details from the Actions menu.
The View Details pane is displayed.
Click OK
Delete files and folders
To delete a file or folder:
From the “Your Files” pane, select the file or folder to delete. Use the Ctrl key to select multiple files.
Select Delete from the Actions menu. The Delete confirmation pane opens.
Click Delete to confirm
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
32
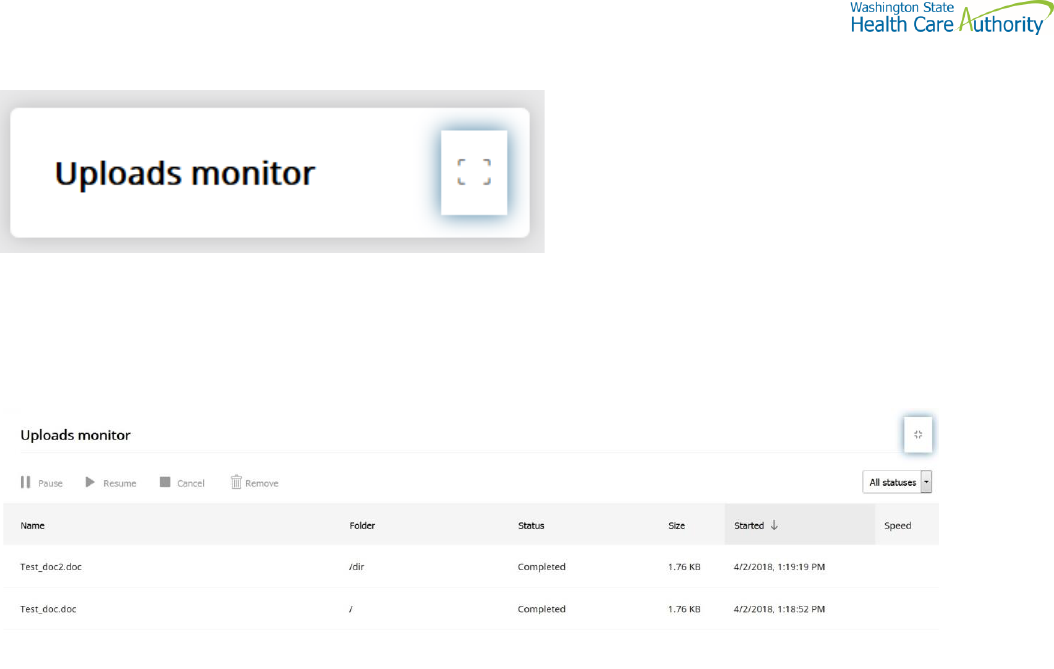
Uploads monitor Page
Monitor uploads
At the bottom of the “Your Files” pane, click Uploads monitor. The Uploads monitor pane is displayed:
Information Displayed
The current status of the file uploads
The progress of each upload if in upload processing
Name of file uploaded/uploading
Folder placement of File
Size of File
Start time & date of Upload
Filter uploads displayed
To filter uploads displayed on the Uploads pane, select the desired filter on the Status drop-down menu.
All statuses
Running
Completed
Paused
Canceled
Failed
Pause uploads

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
33
To pause an upload:
Select uploads you want to pause. Use the Ctrl key to select multiple uploads.
Click Pause.
Resume uploads
To resume an upload:
Select uploads that are paused that you want to resume. Use the Ctrl key to select multiple uploads.
Click Resume.
Cancel uploads
To cancel an upload:
Select the upload that is running that you want to cancel. Use the Ctrl key to select multiple uploads.
Click Cancel.
Remove display entries
To cancel an upload:
Select the upload that is running that you want to cancel. Use the Ctrl key to select multiple uploads.
Click Remove.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
34
Appendix B – SFT Client Options (Partial List)
SFT Client Options – Partial List of
WaTech supported clients-
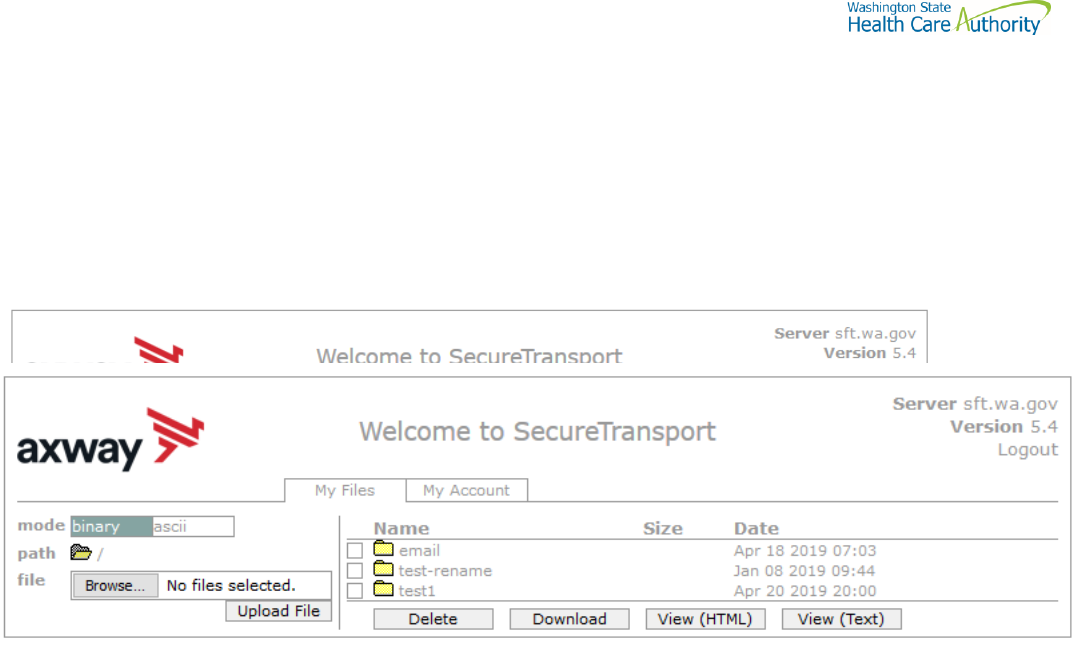
Default browser client
Here is the screen after successful login-
Upload a file by selecting “Browse” tab
Select a file and hit the “Open” tab
The file will appear to the right of the Browse tab.
Select the “Upload File” tab
The file name will be displayed.
Download a file
Check the box to left of your file to download.
Select the “Download” tab
Please do not download a file by selecting the “View” tabs. As you may not get a complete file downloaded.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
35
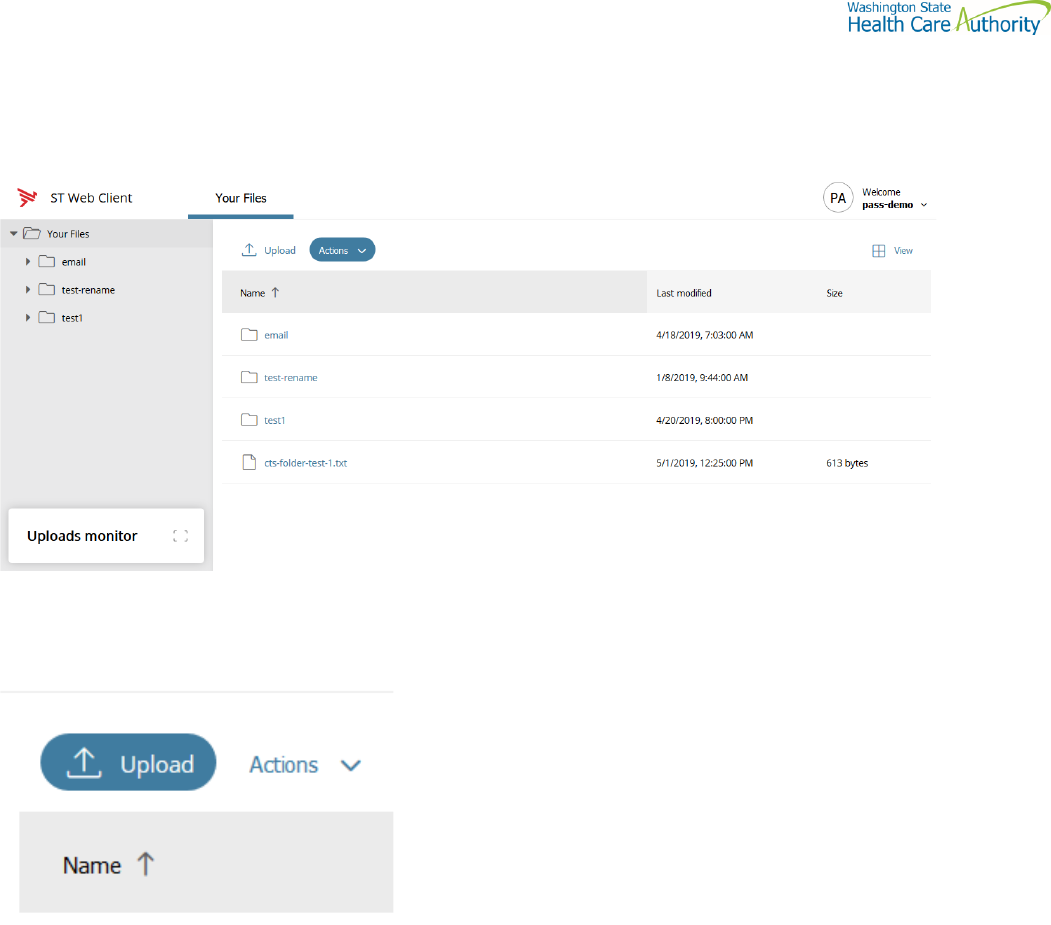
Enhanced Browser Client
After entering your credentials in the default client above, if your account is assigned the ST Web Client, this screen
will appear:
Upload a file by selecting “Upload” tab
Your local folders will be displayed (It defaults to your last location)
Select a file and hit the “Open” tab and this completes the operation of upload. You will get some information on the
screen in regards to the file transfer.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
36
Download a file by
On the screen highlight the file you want to download.
Click on “Actions” drop down will appear, select “Download”
Optional Clients
WaTech does not support any third party client or provide technical support.
WinSCP – With Basic setup information and requirements
URL and Port requirements-
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
37
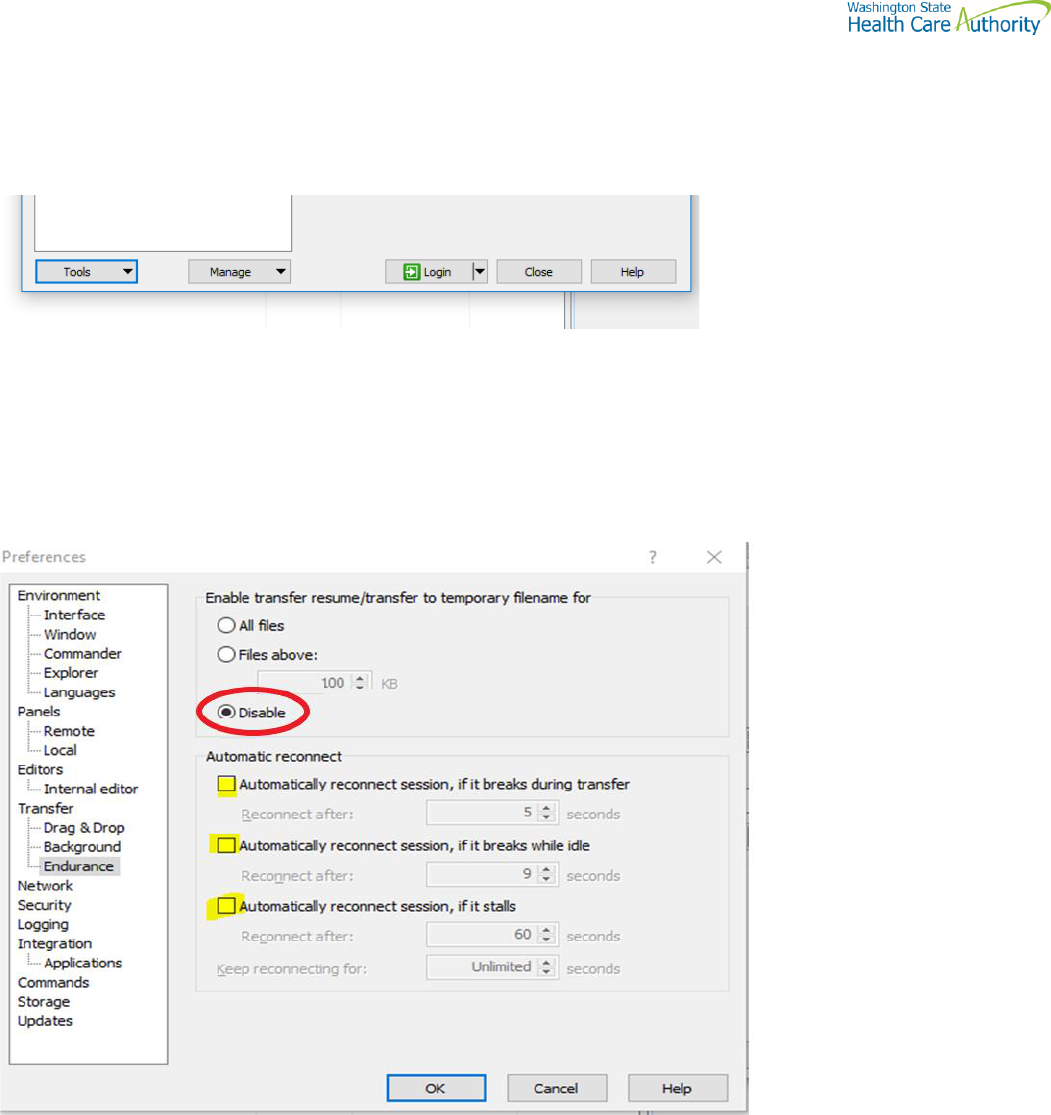
WinSCP – With Basic setup information and requirements – cont’d
Setting requirement to work with SFT. Need to Disable
On the right-hand corner of the Login pop up, select the “Tools” tab
Click on “Endurance” tab and disable the resume feature circled in red.
The yellow highlight is your choice of operation.
Drug Price Transparency Manufacturer Data Submission Guide version 2.0
38
FileZilla- Basic information
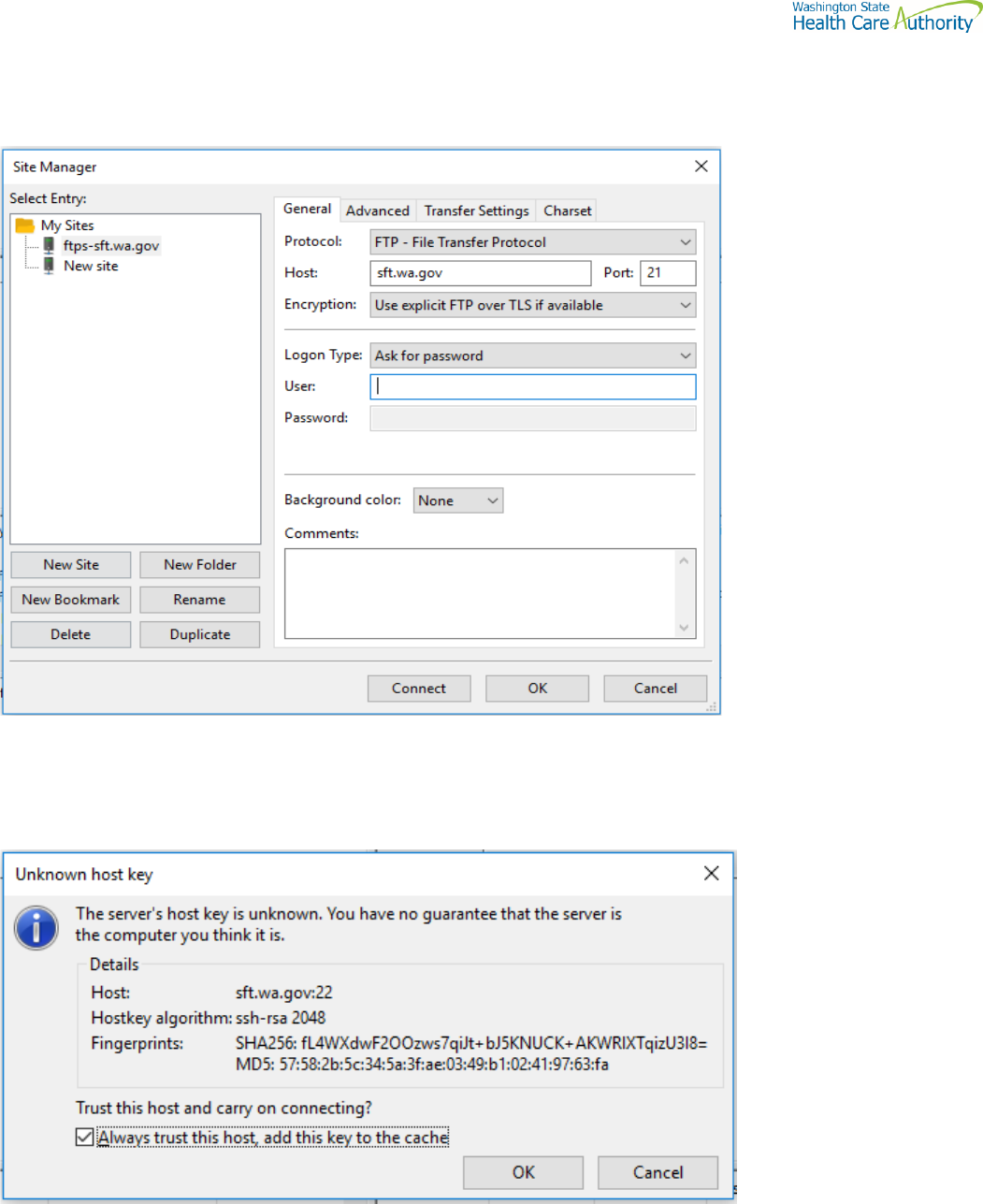
Using FTPS
If using ssh/sftp port 22 need to accept the key on initial login.

Drug Price Transparency Manufacturer Data Submission Guide version 2.0
39
Other client information
General
SFT is expected to work properly with any client or server software which complies with:
RFC 959, RFC 2228, RFC 2389, RFC 2428, RFC 2640, RFC 4217, MD5 Command Extensions,
MFxx Command Extensions for FTP transfers
RFC 4251, RFC 4252, RFC 4253, RFC 4254, Draft RFC - Secure
Shell File Transfer Protocol, Draft RFC - SSH File Transfer Protocol draft-ietf-secsh-filexfer-04.txt for SFTP and SCP
transfers.
List of certified client software by the vendor for file exchange
Software Versions Protocols
cURL 7.58.0 FTPS, HTTPS
CuteFTP Professional 9.2.0.8 (Windows) FTPS
LFTP 4.8.3 FTPS
PSCP (PuTTY) 0.70 SSH
PSFTP (PuTTY SFTP) 0.70 SSH
SmartFTP Client 9.0.2558.0 FTPS
Tectia SSH Client 6.4.15 SSH
VanDyke SecureFX 8.3 SSH
WGET 1.13 FTPS, HTTPS

